Bohr Model For Silicon
0:00 / 2:13 How to Draw the Bohr-Rutherford Diagram of Silicon chemistNATE 258K subscribers Subscribe Subscribed 245 22K views 4 years ago Silicon has 2 electrons in its first shell, 8 in its.
Symbol and electron diagram for silicon Royalty Free Vector
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable.
Silicon Atom Bohr Model Cartoon Style Stock Vector (Royalty Free
Silicon (Si) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Silicon electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.
Silicon Atomic Number Bohr Model Chemical Element, PNG, 1000x1000px
The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915.
Bohr Model Silicon Atom Electron Structure Stock Vector (Royalty Free
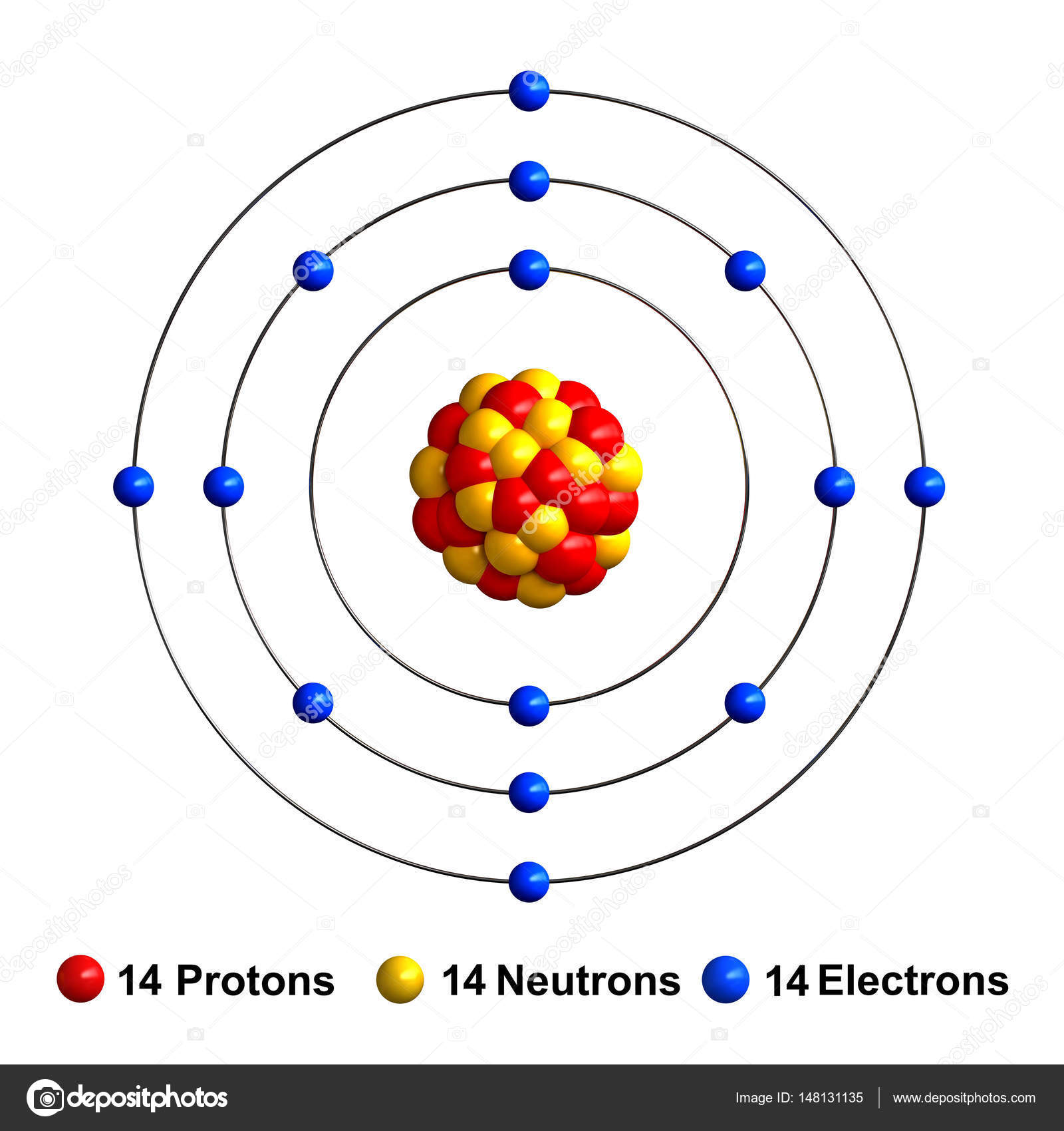
Name: Silicon Symbol: Si Atomic Number: 14 Atomic Mass: 28.0855 amu Melting Point: 1410.0 °C (1683.15 K, 2570.0 °F) Boiling Point: 2355.0 °C (2628.15 K, 4271.0 °F) Number of Protons/Electrons: 14 Number of Neutrons: 14 Classification: Metalloid Crystal Structure: Cubic Density @ 293 K: 2.329 g/cm 3 Color: grey Atomic Structure
Konsep Populer 19+ 1 Atome De Silicium
In this video we'll look at the atomic structure and Bohr model for the Silicon atom (Si). We'll use a Bohr diagram to visually represent where the electrons.
Silicon Bohr Model Diagram, Steps To Draw Techiescientist
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Silicon Si (Element 14) of Periodic Table Elements FlashCards
Silicon is a semiconductor and copper is a conductor. Bohr diagrams of the silicon atom and the copper atom are shown in Fig. 4. Figure 4: Bohr diagrams of the silicon and copper atoms. The core of the silicon atom has a net charge of 4 (14 p +, 10 e-) and the core of the copper atom has a net charge of 1 (29 p +, 28 e-). The core includes.

Image result for silicon atomic model
On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves.

WebElements Periodic Table » Silicon » properties of free atoms
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
silicon atomic model 3d model of silicon atom QFB66
Simplified Bohr model of a silicon atom, with one valence electron excited to the 1st excited energy level. As the diagram shows, the excited electron leaves behind an 'electron-hole' in the valence energy level.Through various mechanisms, this excited electron will lose energy, fall back to the valence energy level, and recombine with its electron-hole.

Bohr model diagram of Silicon Si in atomic physics Stock Vector Adobe
Silicon Bohr Model: Diagram, Steps To Draw Silicon is a group 14 element and is denoted by the symbol Si. It has a high affinity towards oxygen due to which it rarely occurs in its pure form in nature. It is a metalloid and a non-metal. It is the second most abundant mineral found on the earth's surface.
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Silicon Electron Configuration Orbital Diagram For Silicon (Si)
Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons
What is the electron configuration of silicon?
January 2, 2024 by Deep The information on this page is fact-checked. Silicon Bohr model In the silicon Bohr model, the nucleus holds 14 protons and 14 neutrons. Encircling this nucleus are three electron shells, holding a total of 14 electrons. To draw the silicon Bohr model, represent the 14 protons, 14 neutrons, and 14 electrons.
Bohr Model Labeled
Here, we will draw the Bohr diagram of the Silicon atom with some simple steps. Steps to draw the Bohr Model of Silicon atom 1. Find the number of protons, electrons, and neutrons in the Silicon atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.